Jordan joins the Global Platform for Access to Childhood Cancer Medicines
24 September 2024, Amman, Jordan – In a signing ceremony today, the Government of Jordan, represented by the Ministry of Health (MoH), and World Health Organization (WHO) announced that Jordan has become the first Member State in the Eastern Mediterranean Region to join the Global Platform for Access to Childhood Cancer Medicines.
24 September 2024, Amman, Jordan – In a signing ceremony today, the Government of Jordan, represented by the Ministry of Health (MoH), and World Health Organization (WHO) announced that Jordan has become the first Member State in the Eastern Mediterranean Region to join the Global Platform for Access to Childhood Cancer Medicines.
The platform, co-founded by St Jude Children’s Research Hospital and WHO, was launched in 2021 and aims to provide an uninterrupted supply of quality-assured cancer medicines to low- and middle-income countries. Jordan’s membership will ensure an uninterrupted supply of quality-assured medicines for children with cancer, including refugees, free of cost for the next 5 years.
The Global Platform is co-chaired by WHO and St Jude, with UNICEF serving as the partner responsible for the procuring of medicines and delivery to countries.
WHO will continue to provide technical support, in collaboration with the MoH and national stakeholders, to strengthen national supply chain management systems and pharmacy services, enhance the clinical management of childhood cancer, improve national regulatory and monitoring systems and ensure the most effective distribution of medicines to health care facilities.
Jordan’s Minister of Health H.E. Professor Feras Hawari underlined the importance of Jordan’s joining the initiative: “Today marks another win for Jordan and its health system. This agreement will improve access to essential cancer medications for around 500 children diagnosed with cancer each year across the country, guaranteeing that no child with cancer is left behind and contributing to achieving universal health coverage in Jordan”.
Dr Carlos Rodriguez-Galindo, executive vice president of the St Jude Children’s Research Hospital and co-chair of the Global Platform Steering Committee, said: “We are so pleased to be working with Jordan to implement the Global Platform for Access to Childhood Cancer Medicines. The leadership and collaboration among policy makers, health professionals, regulatory authorities, and many other stakeholders to co-design a model that ensures children in Jordan receive the best quality cancer care is critical to the success of the Global Platform and will serve as an inspiration to other countries expected to join the Global Platform in the coming years”.
During the ceremony, WHO Representative to Jordan Dr Jamela Al-Raiby said: “By joining this Global Platform, Jordan vividly demonstrates its commitment to the health and well-being of its children through enhancing access to essential quality-assured childhood cancer medications. WHO commends the Ministry of Health for its leadership and the efforts of national and global stakeholders to enact this pioneering initiative”.
The first batch of medicines is expected to arrive in Jordan by the first quarter of 2025, for use in the 3 major health institutions – King Hussein Cancer Center (KHCC), Royal Medical Services (RMS) and King Abdullah University Hospital – that provide childhood cancer care.
National stakeholders, with the support of the Global Platform and WHO Jordan Country Office, will collaborate to create an implementation plan to assure the safe and effective delivery and distribution of the medicines.
Note to editors
The Global Platform for Access to Childhood Cancer Medicines was launched in 2021. St Jude Children’s Research Hospital and WHO jointly developed the Global Platform, a complement to the Global Initiative for Childhood Cancer, with UNICEF and the Pan American Health Organization Strategic Fund serving as the procurement agents. Between 2022 and 2027, the Global Platform aims to provide an uninterrupted supply of quality-assured cancer medicines to approximately 120 000 children in low- and middle-income countries, with the expectation of scaling up supplies in future years. The Platform provides end-to-end support in consolidating global demand to shape the market and assists countries with the selection of medicines, development of treatment standards and the building of information systems to track that effective care is being provided.
New products

Device for aspiration of mucus congesting the upper airways of the newborn or for suctioning tracheal / bronchial secretions (of newborns, children and adults) which will be analysed in laboratory.
See more
Safety Scalpel has a reusable stainless steel handle similar in weight and balance to traditional metal handle scalpels, providing surgeons and techs with necessary safety features without losing the familiarity, control and ease-of-use of their current scalpels.
See more
Reusable Stainless Steel Handles with a measure or graduation on the reverse side to assist with training, marking of the correct surgical site and trauma injury investigations. Fitment No.3. for Blades - No.6, 9, 10, 10A, 10R, E/11, 11, 11P, 12, 12D, 13, 14, 15, 15A, 15C, 15T, 16, 40 and SG3.
See more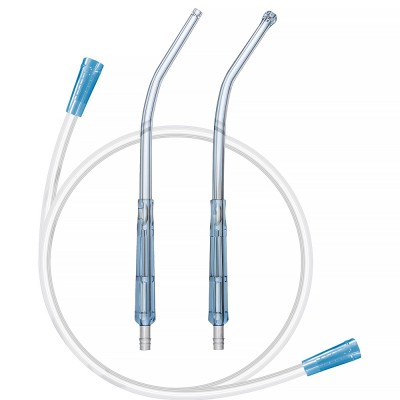
Yankauer Suction Set is used for suctioning of oropharyngeal secretion or clears the operative site like fluid, blood or pus from a wound or body cavity.
See more
Closed Wound Suction Unit is an effective device for closed wound drainage under negative pressure post operatively. Made from non toxic, non-irritant PVC.
See more
Used for rapid non operative plueral and Chest drainage. Manufactured from non toxic, medical grade PVC compound.
See more
Oral Airway Kits include a range of sizes in 6, 7, or 8-piece kits for convenience. These oral airways are polypropylene and feature a smooth, non-brittle finish that permits easy insertion with a reinforced bite block to prevent airway collapse.
See more
3-Way Stopcock with Swivel Male Luer Lock are a leading choice of anesthesiologists and critical care workers worldwide.
See more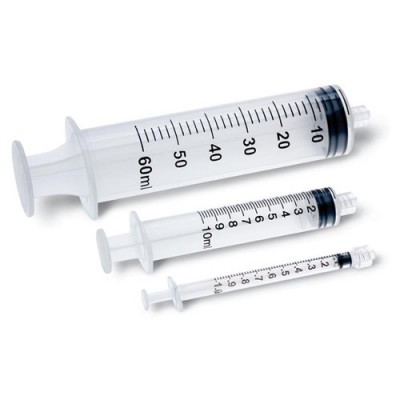
Economy Syringes Without Needle offer easy dosage measurement and observation of air bubbles and flashbacks. These syringes are available in a wide range of sizes and are packaged sterile. These syringes are also latex and pyrogen free.
See more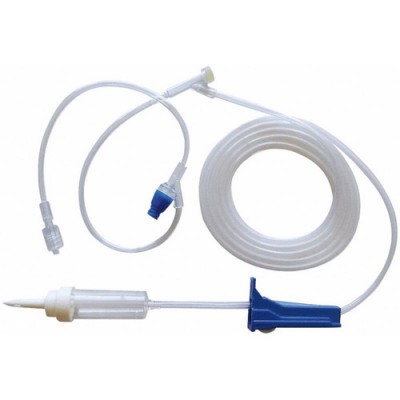
83" 10 Drop/mL Admin Set has a non-vented drip chamber with a roller clamp and split septum Y-site with a needleless PRN and rotary male luer lock. This set is individually packaged and is sterile.
See more
20" Extension Set is designed to control IV fluid and medications using a 'regulator dial' to control the rate of infusion. This extension set has a pre-pierced injection Y-site with a slide clamp and a rotating male leur lock and is latex-free.
See more
Extension Set with Sure-Lok Connector has a needleless Sure-Lok connector with a rotating male leur lock. It is non-pyrogenic and DEHP and Latex-free. This extension set is packaged sterile.
See more
